Setrusumab (UX-143)
For the potential treatment of Osteogenesis Imperfecta (“OI”), a rare genetic bone condition affecting children and adults.

We have already delivered five successful Phase 2 studies and a Phase 1b/2 ongoing. Our pipeline currently consists of six clinical-stage product candidates. Click below to see the relevant scientific publications for our products.

For the potential treatment of Osteogenesis Imperfecta (“OI”), a rare genetic bone condition affecting children and adults.

For the potential treatment of severe Alpha-1 Antitrypsin Deficiency-associated Lung Disease (AATD-LD) and Bronchiolitis Obliterans Syndrome (BOS).

For the potential treatment of patients with advanced or metastatic
solid tumors.
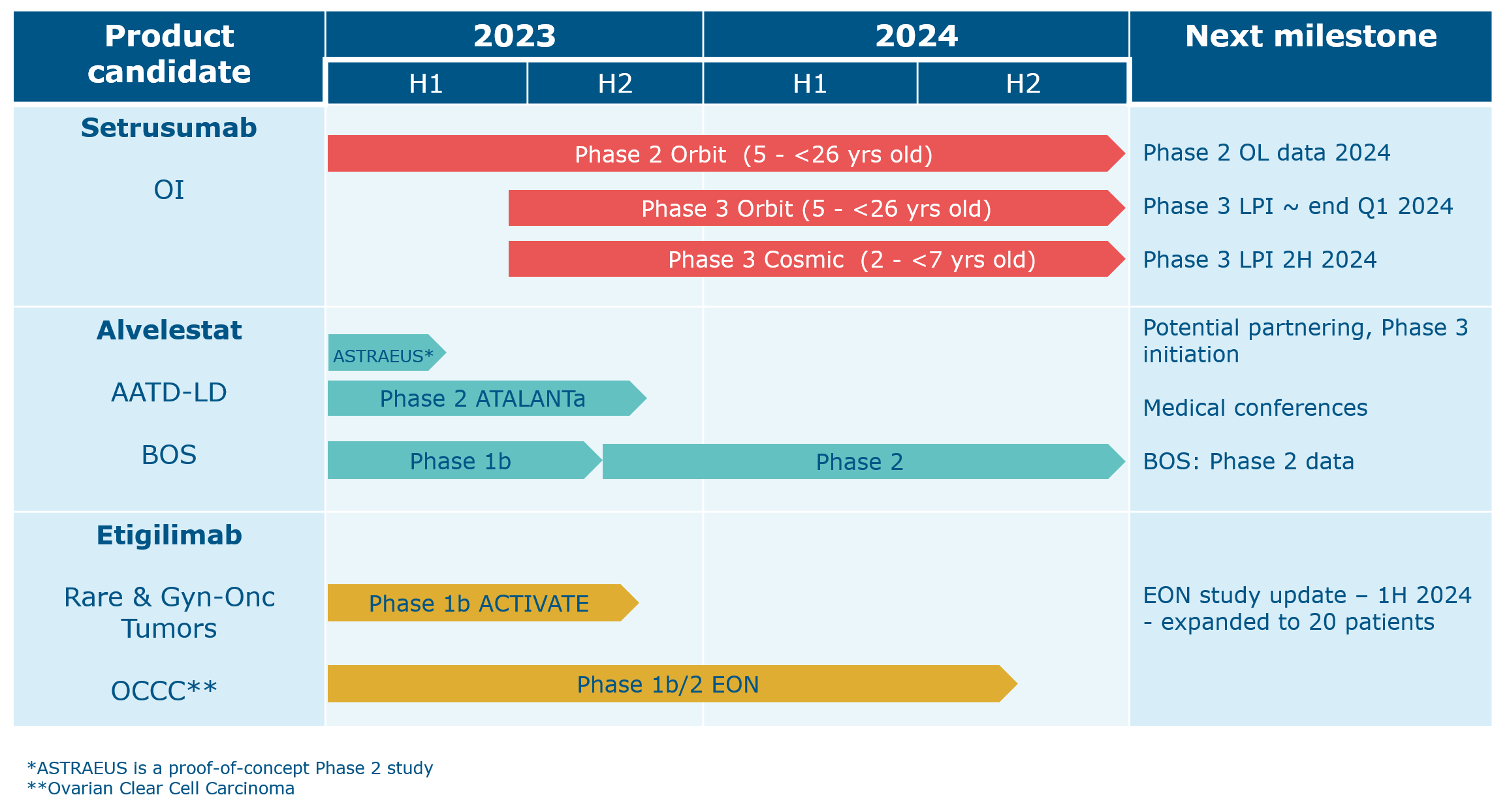
We have one additional program with current partnering opportunities. Navicixizumab has been licensed out to OncXerna and leflutrozole has been licensed to ReproNovo.

For the potential treatment of platinum-resistant ovarian cancer, partnered with OncXerna Therapeutics Inc. for further development.

For the potential treatment of acute exacerbations of chronic obstructive pulmonary disease (“AECOPD”).

A novel once weekly oral aromatase inhibitor. Partnered with ReproNovo for further development.

London, March 26, 2025 – Mereo BioPharma Group plc (NASDAQ: MREO) (“Mereo” or the “Company”), a clinical-stage biopharmaceutical company focused on rare diseases, today announced its financial results for the full year ended December 31, 2024, and provided recent corporate highlights.

NOVATO, Calif., October 7, 2024 – Ultragenyx Pharmaceutical Inc. (NASDAQ: RARE), a biopharmaceutical company focused on the development of novel products for rare and ultra-rare diseases, today announced that it has received Breakthrough Therapy Designation from the U.S. Food and Drug Administration (FDA) for setrusumab (UX143) as a treatment to reduce the risk of fracture associated with osteogenesis imperfecta (OI) Type I, III, or IV in patients 2 years of age and older.

London, September 12, 2024 - Mereo BioPharma Group plc (NASDAQ: MREO) (“Mereo” or the “Company”), a clinical-stage biopharmaceutical company focused on rare diseases, today announced that Dr. Denise Scots-Knight, Chief Executive Officer, will participate in a Fireside Chat at the 2024 Cantor Global Healthcare Conference on Wednesday, September 18, 2024, at 10:55am ET / 03:55pm BST.

NOVATO, Calif. and LONDON, UK — June 11, 2024 — Ultragenyx Pharmaceutical Inc. (NASDAQ: RARE) and Mereo BioPharma Group plc (NASDAQ: MREO) today announced positive 14-month results from the Phase 2 portion of the ongoing Phase 2/3 Orbit study (NCT05125809) demonstrating that, as of a May 24, 2024 data cut-off date, treatment with setrusumab (UX143) continued to significantly reduce incidence of fractures in patients with OI with at least 14 months of follow-up. Treatment with setrusumab also resulted in ongoing and meaningful improvements in lumbar spine bone mineral density (BMD) at month 12 without evidence of plateau.